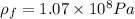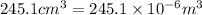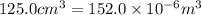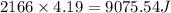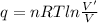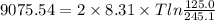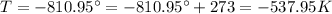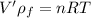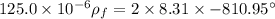Physics, 31.08.2019 03:30 Karinaccccc
An ideal gas is brought through an isothermal compression process. the 2.00 mol of gas goes from having an initial volume of 245.1 cm^3 to 125.0 cm^3. if 2166 cal is released by the gas during this process, what are the temperature t of the gas and the final pressure pf? the gas constant is r=8.31 j/molâk, and there are 4.19 j/cal. t= k
pf = pa

Answers: 1


Another question on Physics

Physics, 22.06.2019 02:10
Which statement correctly describes the relationship between frequency and wavelength?
Answers: 2

Physics, 22.06.2019 18:10
Which of the following statement is false ? a.batteries should never be stored on metal trays or outside in the elements)b, battery acid cannot be neutralized)c.do not stack batteries on top of each other)d.never put a damaged battery in a dumpster. i
Answers: 1

Physics, 22.06.2019 20:50
The second largest public utility in the nation is the sole provider of electricity in 32 counties of southern florida. to meet the monthly demand for electricity in these counties, which is given by the inverse demand function p = 1,200 – 4q, the utility company has set up two electric generating facilities: q1 kilowatts are produced at facility 1, and q2 kilowatts are produced at facility 2 (so q = q1 + q2). the costs of producing electricity at each facility are given by c1(q1) = 8,000 + 6q12 and c2(q2) = 6,000 + 3q22, respectively. determine the profit-maximizing amounts of electricity to produce at the two facilities, the optimal price, and the utility company’s profit
Answers: 3

Physics, 23.06.2019 00:30
What is the largest meteoroid that has collided with this planet
Answers: 1
You know the right answer?
An ideal gas is brought through an isothermal compression process. the 2.00 mol of gas goes from hav...
Questions




Computers and Technology, 13.11.2020 19:50

Business, 13.11.2020 19:50


Mathematics, 13.11.2020 19:50



Law, 13.11.2020 19:50

Computers and Technology, 13.11.2020 19:50

Chemistry, 13.11.2020 19:50

Computers and Technology, 13.11.2020 19:50

History, 13.11.2020 19:50

Social Studies, 13.11.2020 19:50

Mathematics, 13.11.2020 19:50


History, 13.11.2020 19:50

Mathematics, 13.11.2020 19:50

Biology, 13.11.2020 19:50