
Physics, 02.09.2019 22:10 angel13sigala
Amajor component of gasoline is octane . when octane is burned in air, it chemically reacts with oxygen gas to produce carbon dioxide and water . what mass of water is produced by the reaction of of octane

Answers: 3


Another question on Physics

Physics, 22.06.2019 00:10
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3

Physics, 22.06.2019 06:30
At very high pressures, gases become and will eventually a) more dense; become hotter b) more dense; change to a liquid or solid c) less dense; combust d) less dense; turn into a liquid
Answers: 1

Physics, 22.06.2019 09:30
Astone is dropped from a cliff and falls 9.44 meters. what is the speed of the stone when it reaches the ground? a. 13.6 m/sec b. 1.39 m/sec c. 185 m/sec d. 9.80 m/sec
Answers: 3

Physics, 22.06.2019 11:00
Consider a system to be two train cars traveling toward each other. what is the total momentum of the system before the train cars collide? kg • what must the total momentum of the system be after the train cars collide? kg •
Answers: 2
You know the right answer?
Amajor component of gasoline is octane . when octane is burned in air, it chemically reacts with oxy...
Questions


English, 03.08.2019 00:40






Business, 03.08.2019 00:40

Mathematics, 03.08.2019 00:40

Biology, 03.08.2019 00:40

Arts, 03.08.2019 00:40

Computers and Technology, 03.08.2019 00:40

Health, 03.08.2019 00:40

Health, 03.08.2019 00:40

Health, 03.08.2019 00:40

Computers and Technology, 03.08.2019 00:40

Health, 03.08.2019 00:40

Chemistry, 03.08.2019 00:40

Biology, 03.08.2019 00:40

Social Studies, 03.08.2019 00:40

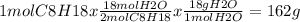 of water per mol of C8H18 burned
of water per mol of C8H18 burned

