
Physics, 04.09.2019 22:10 faithyholcomb
What is the enthalpy change (in kj) of a chemical reaction that raises the temperature of 250.0 ml of solution having a density of 1.25 g/ml by 3.33 °c? (the specific heat of the solution is 3.74 joules/gram-k.) what is the enthalpy change (in kj) of a chemical reaction that raises the temperature of 250.0 ml of solution having a density of 1.25 g/ml by 3.33 °c? (the specific heat of the solution is 3.74 joules/gram-k.) -3.89 -7.43 8.20 6.51 -12.51

Answers: 3


Another question on Physics

Physics, 21.06.2019 16:40
Three point charges, two positive and one negative, each having a magnitude of 20 μc are placed at the vertices of an equilateral triangle (30 cm on a side). what is the magnitude of the electrostatic force on the negative charge
Answers: 3


Physics, 22.06.2019 11:30
Water is siphoned from a large tank and discharges into the atmosphere through a 50-mm diameter tube. the end of the tube is b = 2.1 m below the tank bottom which is a = 7.4 m deep, and viscous effects are negligible. determine the maximum height h over which the water can be siphoned without cavitation occurring. atmospheric pressure is 101.4 kpa, and the water vapor pressure is 1.79 kpa (absolute)
Answers: 3

Physics, 22.06.2019 19:00
Abatter hits a 0.140-kg baseball that was approaching him at 30 m/s and, as a result, the ball leaves the bat at 40 m/s in the reverse of its original direction. the ball remains in contact with the bat for 2.0 ms. what is the magnitude of the average force exerted by the bat on the ball?
Answers: 1
You know the right answer?
What is the enthalpy change (in kj) of a chemical reaction that raises the temperature of 250.0 ml o...
Questions

Biology, 29.08.2019 22:30



History, 29.08.2019 22:30




Mathematics, 29.08.2019 22:30


Mathematics, 29.08.2019 22:30



History, 29.08.2019 22:30

Social Studies, 29.08.2019 22:30





Mathematics, 29.08.2019 22:30

Chemistry, 29.08.2019 22:30

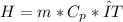
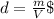 ⇒
⇒ 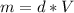
![m=250[mL]*1,25[\frac{g}{mL}]=312,5 [g]](/tpl/images/0222/9306/18a07.png)
![H=312,5*3,74*(-3,33)=-3891 [J]=-3,81[kJ]](/tpl/images/0222/9306/684a5.png)


