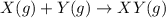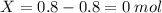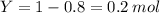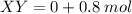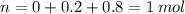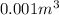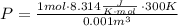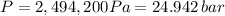Consider the gaseous reaction, x(g) + y(g) →xy(g) a mixture of 0.8 mole of gas x and 1 mole of gas y was allowed to react at 300 k and 1 l sealed container. at the end of the reaction, what is the total pressure of the mixture? the temperature and volume remains constant during the reaction.

Answers: 3


Another question on Physics

Physics, 22.06.2019 06:00
The magnetic field inside a superconducting solenoid is 4.50 t. the solenoid has an inner diameter of 6.20 cm and a length of 26.0 cm. determine (a) the magnetic energy density in the field and (b) the energy stored in the magnetic field within the solenoid.
Answers: 2

Physics, 22.06.2019 09:10
Which lists the organs in the correct order as food passes from the mouth to anus?
Answers: 1

Physics, 22.06.2019 09:30
Along the line connecting the two charges, at what distance from the charge q1 is the total electric field from the two charges zero? express your answer in terms of some or all of the variables s, q1, q2 and k =14ï€ïµ0. if your answer is difficult to enter, consider simplifying it, as it can be made relatively simple with some work.
Answers: 3

You know the right answer?
Consider the gaseous reaction, x(g) + y(g) →xy(g) a mixture of 0.8 mole of gas x and 1 mole of gas y...
Questions


Biology, 28.01.2020 09:31

Biology, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31

Social Studies, 28.01.2020 09:31



English, 28.01.2020 09:31



Health, 28.01.2020 09:31

Business, 28.01.2020 09:31

Social Studies, 28.01.2020 09:31



Biology, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31



Physics, 28.01.2020 09:31

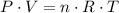
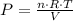
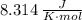 in SI units)T is the absolute TemperatureV is the volume
in SI units)T is the absolute TemperatureV is the volume