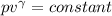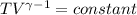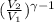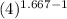Three kilograms of argon (ar) changes from an initial volume and a temperature of 298k to (a) four times the volume and a temperature of 298k and (b) one fourth the original volume under adiabatic conditions. in each case: calculate the amount of work performed. is the work done by the system or the surroundings? (pl/, for monatomic gases is 1.667)

Answers: 2


Another question on Physics

Physics, 22.06.2019 10:30
If y gets smaller as x gets bigger and y have an relationship?
Answers: 1

Physics, 22.06.2019 13:00
4. if you were an astronaut on the moon, what would you experience? what would you see from your perspective?
Answers: 1

Physics, 22.06.2019 17:50
Which of the following best describes internal energy? a. the difference between the kinetic and potential energies of the particles in a system b. the sum of the kinetic and potential energies of the particles in a system c. the sum of the kinetic and thermal energies of the particles in a system d. the difference between the kinetic and thermal energies of the particles in a system
Answers: 2

Physics, 22.06.2019 20:30
Atypical jetliner lands at a speed of 146 mi/h and decelerates at the rate of (10.4 mi/h)/s. if the jetliner travels at a constant speed of 146 mi/h for 1.5 s after landing before applying the brakes, what is the total displacement of the jetliner between touchdown on the runway and coming to rest?
Answers: 2
You know the right answer?
Three kilograms of argon (ar) changes from an initial volume and a temperature of 298k to (a) four t...
Questions

Mathematics, 08.05.2021 01:00









Chemistry, 08.05.2021 01:00

Biology, 08.05.2021 01:00

Mathematics, 08.05.2021 01:00


Mathematics, 08.05.2021 01:00


Mathematics, 08.05.2021 01:00


Mathematics, 08.05.2021 01:00

Mathematics, 08.05.2021 01:00

Biology, 08.05.2021 01:00