
Physics, 07.09.2019 01:30 chloedfite2978
Derive the velocity of an electron in a hydrogen atom using bohr's model. the electron has a mass of m, charge of (-e) and an orbit radius of r. make sure you explain all the teps including quantization of angular momentum to get full credits

Answers: 3


Another question on Physics

Physics, 21.06.2019 17:30
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j ?
Answers: 2

Physics, 22.06.2019 01:30
The passing of heat through a material while the material itself stays in place. a. radiation b. conduction c. convection
Answers: 2

Physics, 22.06.2019 21:10
For the below questions, consider a consumer that consumes two goods, x and z with the following utility function. u with bar on top space equals space x to the power of 1 third end exponent z to the power of 2 over 3 end exponent suppose initial values for income and the prices of goods x and z are y equals 90, p subscript x equals space 10, and p subscript z equals 15 respectively, then the price of good x falls to syntax error from line 1 column 89 to line 1 column 100. unexpected '\'.. what is the magnitude of the total effect
Answers: 3

Physics, 22.06.2019 23:30
The power exerted by a muscle is the product of the force exerted and the velocity of contraction. the area of which of these shaded regions represents the power exerted while a weight is lifted at maximum speed?
Answers: 2
You know the right answer?
Derive the velocity of an electron in a hydrogen atom using bohr's model. the electron has a mass of...
Questions






Computers and Technology, 21.05.2020 03:05


History, 21.05.2020 03:05

Chemistry, 21.05.2020 03:05







Mathematics, 21.05.2020 03:05

English, 21.05.2020 03:05

History, 21.05.2020 03:05

Chemistry, 21.05.2020 03:05

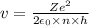
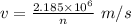
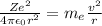 ......1
......1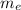 is the mass of the electron
is the mass of the electron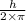 .
.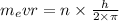 ....2
....2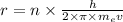
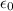 = 8.854×10⁻¹² C² N⁻¹ m⁻²
= 8.854×10⁻¹² C² N⁻¹ m⁻²

