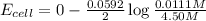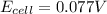Answers: 1


Another question on Physics

Physics, 20.06.2019 18:04
The graph shown is a velocity-time graph of an object. what is the acceleration of the object from 6.0 to 10.0 seconds?
Answers: 1

Physics, 22.06.2019 05:30
The a992 steel rod bc has a diameter of 50 mm and is used as a strut to support the beam. determine the maximum intensity w of the uniform distributed load that can be applied to the beam without risk of causing the strut to buckle. take f.s. = 2 against bucklin
Answers: 3

Physics, 22.06.2019 11:50
The mass of the sun is 1.99×1030kg and its distance to the earth is 1.50×1011m. what is the gravitational force of the sun on the earth?
Answers: 3

Physics, 22.06.2019 15:30
How many neutrons does element x if it’s atomic number is 28 and its mass number is 87
Answers: 1
You know the right answer?
Avoltaic cell is constructed with two zn2+-zn electrodes, where the half-reaction is zn2+ + 2e− → zn...
Questions







Mathematics, 16.07.2019 02:00

Mathematics, 16.07.2019 02:00



Mathematics, 16.07.2019 02:00


Mathematics, 16.07.2019 02:00




Biology, 16.07.2019 02:00


English, 16.07.2019 02:00

History, 16.07.2019 02:00

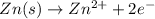
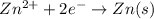
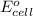 is equal to zero.
is equal to zero.![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}{diluted}}{[Zn^{2+}{concentrated}]}](/tpl/images/0229/3637/38b09.png)
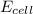 = ?
= ?![[Zn^{2+}{diluted}]](/tpl/images/0229/3637/d7205.png) = 0.0111 M
= 0.0111 M![[Zn^{2+}{concentrated}]](/tpl/images/0229/3637/890cc.png) = 4.50 M
= 4.50 M