

Answers: 3


Another question on Physics

Physics, 21.06.2019 18:30
An experiment is designed to test the relationship between the initial height of a basketball before it is dropped to the height of its rebound bounce. the height of the rebound bounce is measured using a scale positioned behind the ball. in the above experiment, which condition would not be controlled? a). the starting height of the ballb). the surface the ball bounces on c). the type of ball d). the method to measure the rebound height of the ball
Answers: 1

Physics, 22.06.2019 18:30
Aprotein molecule in an electrophoresis gel has a negative charge. the exact charge depends on the ph of the solution, but 30 excess electrons is typical. what is the magnitude of the electric force on a protein with this charge in a 1600n/c electric field? write in two significant numbers, and also in newton
Answers: 2

Physics, 22.06.2019 19:00
According to an observer on earth, planet x is stationary. maria and samir depart earth on their spaceships at the same time, each flying at 0.5c. maria flies toward planet x and samir flies in the opposite direction. according to maria, her voyage to planet x takes 9 years. how many years does maria's voyage take according to samir?
Answers: 3

Physics, 23.06.2019 01:00
What will happen in a hybrid vehicle if current flow becomes too high due to a short?
Answers: 1
You know the right answer?
An engineer examining the oxidation of so 2 in the manufacture of sulfuric acid determines that kc =...
Questions

Mathematics, 15.01.2021 22:00





Biology, 15.01.2021 22:00

Mathematics, 15.01.2021 22:00


Mathematics, 15.01.2021 22:00

Mathematics, 15.01.2021 22:00


Law, 15.01.2021 22:00


Mathematics, 15.01.2021 22:00

Mathematics, 15.01.2021 22:00

Health, 15.01.2021 22:00



Mathematics, 15.01.2021 22:00

Mathematics, 15.01.2021 22:00

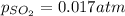

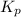 with
with 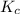 is given by the formula:
is given by the formula: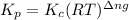

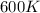
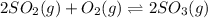
 = change in number of moles of gas particles =
= change in number of moles of gas particles = 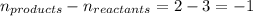

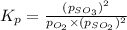
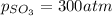
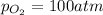
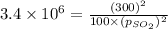
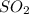 at equilibrium is 0.017 atm.
at equilibrium is 0.017 atm.

