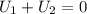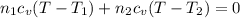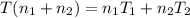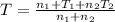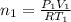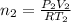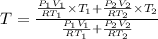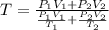Physics, 14.09.2019 07:20 jamalnellum56
Arigid adiabatic container is divided into two parts containing n1 and n2 mole of ideal gases respectively, by a movable and thermally conducting wall. their pressure and volume are p1, v1 for part 1 and p2, v2 for part 2 respectively. find the final pressure p and temperature t after the two gas reaches equilibrium. assume the constant volume specific heats of the two gas are the same.

Answers: 2


Another question on Physics

Physics, 22.06.2019 16:00
In which of the following is positive work done by a person on a suitcase
Answers: 1

Physics, 22.06.2019 17:00
How much energy is supplied to each coulomb of charge that flows through a 12-v battery?
Answers: 1

Physics, 22.06.2019 23:00
Determine the force the sun exerts on an object with a mass of 80.0 kg if that object is on the earth. what is the force exerted by the moon on the same object? what is the force the earth exerts on it?
Answers: 1

Physics, 23.06.2019 01:20
Afisherman notices that his boat is moving up and down periodically, owing to waves on the surface of the water. it takes a time of 2.70 s for the boat to travel from its highest point to its lowest, a total distance of 0.700 m. the fisherman sees that the wave crests are spaced a horizontal distance of 6.50 m apart. a. how fast are the waves traveling? b. what is the amplitude a of each wave?
Answers: 1
You know the right answer?
Arigid adiabatic container is divided into two parts containing n1 and n2 mole of ideal gases respec...
Questions

Mathematics, 14.04.2020 21:39


Mathematics, 14.04.2020 21:39




Social Studies, 14.04.2020 21:39












Biology, 14.04.2020 21:39

History, 14.04.2020 21:39

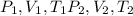
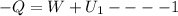
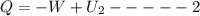
 change in internal Energy of gas
change in internal Energy of gas