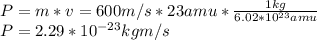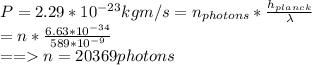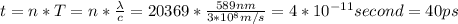Physics, 14.09.2019 08:30 emilylunaa
Asodium atom will absorb light with a wavelength near 589 nm if the light is within 10 mhz of the resonant frequency. the atomic mass of sodium is 23. (i) calculate the number of "yellow" photons of wavelength 2 = 589 nm that must be absorbed to stop a sodium atom initially at room temperature (v-600 m/s). [7 marks] (ii) what is the minimum time needed to cool a sodium atom?

Answers: 3


Another question on Physics

Physics, 21.06.2019 14:40
A50.0-n box is sliding on a rough horizontal floor, and the only horizontal force acting on it is friction. you observe that at one instant the box is sliding to the right at and that it stops in 2.25 s with uniform acceleration. what magnitude force does friction exert on this box
Answers: 1

Physics, 21.06.2019 21:10
Tafari worked one summer on a ship that set weather buoys in the ocean. he watched how one of the buoys moved in the water. describe which parts of the wave would cause the buoy to bob up and down. which wave property determined how fast the buoys bobbed in the water? he observed that when the wind blew harder, the ocean waves were larger, and the buoys moved away from the ship. what effect, if any, did the waves have on how far the buoys moved? explain your answer.
Answers: 3

Physics, 22.06.2019 04:10
Atotal charge of –6.50 µc is uniformly distributed within a sphere that has a radius of 0.150 m. what is the magnitude and direction of the electric field at 0.300 m from the surface of the sphere? a) 2.89 × 105 n/c, radially inward b) 6.49 × 105 n/c, radially outward c) 4.69 × 105 n/c, radially inward d) 9.38 × 105 n/c, radially outward e) 1.30 × 106 n/c, radially inward
Answers: 3

You know the right answer?
Asodium atom will absorb light with a wavelength near 589 nm if the light is within 10 mhz of the re...
Questions

English, 07.04.2020 23:55

Mathematics, 07.04.2020 23:55





Computers and Technology, 07.04.2020 23:55


History, 07.04.2020 23:55



Computers and Technology, 07.04.2020 23:55

Mathematics, 07.04.2020 23:55

Mathematics, 07.04.2020 23:55




English, 07.04.2020 23:55