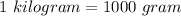Physics, 14.09.2019 08:30 4804174946
Represent 0.783 kg with sl units having an appropriate prefix express your answer to three significant figures and include the appropriate units. p: a value ro ? units 0.783 kg

Answers: 3


Another question on Physics

Physics, 22.06.2019 00:30
Consider an ordinary, helium-filled party balloon with a volume of 2.2 ft3. the lifting force on the balloon due to the outside air is the net resultant of the pressure distribution exerted on the exterior surface of the balloon. using this fact, we can derive archimedes’ principle, namely that the upward force on the balloon is equal to the weight of the air displaced by the balloon. assuming that the balloon is at sea level, where the air density is 0.002377 slug/ft3, calculate the maximum weight that can be lifted by the balloon. note: the molecular weight of air is 28.8 and that of helium is 4.
Answers: 2

Physics, 22.06.2019 01:30
The transfer of heat through the movement of a gas or liquid a. convection b. conduction c. radiation
Answers: 2

Physics, 22.06.2019 08:00
Ms.hidalgo opens the door to her classroom. her classroom is 60 degrees and the air outside is 80. predict what will happen using your knowledge of how heat flows.
Answers: 2

Physics, 22.06.2019 18:50
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3
You know the right answer?
Represent 0.783 kg with sl units having an appropriate prefix express your answer to three significa...
Questions

English, 22.12.2021 18:00



English, 22.12.2021 18:00

SAT, 22.12.2021 18:00



Mathematics, 22.12.2021 18:10



History, 22.12.2021 18:10




Mathematics, 22.12.2021 18:10

Social Studies, 22.12.2021 18:10

English, 22.12.2021 18:10