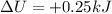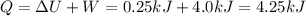Physics, 18.09.2019 03:20 tommyewall34
Agas contained within a piston-cylinder assembly, initially at a volume of 0.1 m3 , undergoes a constant-pressure expansion at 2 bars to a final volume of 0.12 m3 , while being slowly heated through the base. the change in internal change of the gas 0.25 kj. the piston and cylinder walls are fabricated from heat-resistant material and the piston moves smoothly in the cylinder. for the gas as the system, evaluate work and heat transfer each in kj (neglect the potential energy change and kinetic energy change).

Answers: 2


Another question on Physics

Physics, 21.06.2019 22:40
Explain vector addition, triangle method and parallelogram method
Answers: 1


Physics, 22.06.2019 03:00
Isla’s change in velocity is 30 m/s, and hazel has the same change in velocity. which best explains why they would have different accelerations? isla had negative acceleration, and hazel had positive. isla had a different time than hazel. isla had positive acceleration, and hazel had negative. isla went a farther distance than hazel.
Answers: 1

You know the right answer?
Agas contained within a piston-cylinder assembly, initially at a volume of 0.1 m3 , undergoes a cons...
Questions


Health, 09.09.2021 02:40

History, 09.09.2021 02:40


Mathematics, 09.09.2021 02:40




Mathematics, 09.09.2021 02:40


English, 09.09.2021 02:40


Mathematics, 09.09.2021 02:40

Mathematics, 09.09.2021 02:40


Mathematics, 09.09.2021 02:40

Biology, 09.09.2021 02:40

Biology, 09.09.2021 02:40


Mathematics, 09.09.2021 02:40

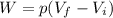
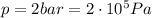 is the pressure
is the pressure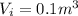 is the initial volume
is the initial volume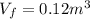 is the final volume
is the final volume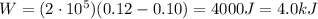
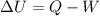
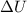 is the change in internal energy of the gas
is the change in internal energy of the gas