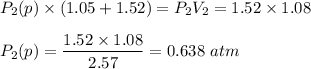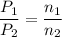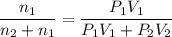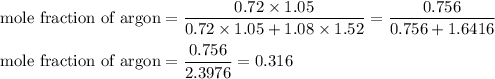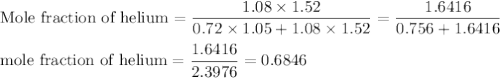A1.05-l bulb and a 1.52-l bulb are connected by a stopcock and filled, respectively, with argon at 0.72 atm and helium at 1.08 atm at the same temperature. calculate the total pressure, the partial pressures of each gas, and the mole fraction of each gas after the stopcock has been opened. assume ideal-gas behavior. (answer in 3 significant figures)

Answers: 3


Another question on Physics

Physics, 21.06.2019 19:10
Natural forces that can alter ecosystems include seasons and climate changes. t or f
Answers: 1

Physics, 22.06.2019 04:30
In a system, when potential energy decreases, then entropy also decreases. true false
Answers: 2


Physics, 22.06.2019 09:00
Abicycle slows down when the rider applies the brakes. what type of energy transformation is involved in this example? a. kinetic energy into heat energy b. heat energy into potential energy c. potential energy into kinetic energy d. kinetic energy into mechanical energy
Answers: 1
You know the right answer?
A1.05-l bulb and a 1.52-l bulb are connected by a stopcock and filled, respectively, with argon at 0...
Questions



Spanish, 28.03.2021 14:00



Mathematics, 28.03.2021 14:00



History, 28.03.2021 14:00

Chemistry, 28.03.2021 14:00

Social Studies, 28.03.2021 14:00

Social Studies, 28.03.2021 14:00

Computers and Technology, 28.03.2021 14:00



Social Studies, 28.03.2021 14:00

English, 28.03.2021 14:00

Mathematics, 28.03.2021 14:00

Mathematics, 28.03.2021 14:00

Computers and Technology, 28.03.2021 14:00

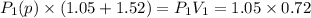
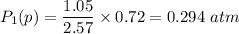
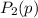 be the partial pressure of gas in bulb B then
be the partial pressure of gas in bulb B then