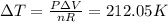When 25.9 j was added as heat to a particular ideal gas, the volume of the gas changed from 41.0 cm3 to 67.5 cm3 while the pressure remained constant at 1.08 atm. (a) by how much did the internal energy of the gas change? if the quantity of gas present is 1.66 x 10-3 mol, find the molar specific heat of the gas at (b) constant pressure and (c) constant volume.

Answers: 3


Another question on Physics

Physics, 21.06.2019 21:00
A150 w lamp emits light of wavelength 590 nm uniformly in all directions. what is the photon flux (photons per unit area per unit time) on a small screen at a distance 2.3 m from the lamp? assume the photons are uniformly distributed over the surface of a sphere of radius 2.3 m.
Answers: 2

Physics, 22.06.2019 11:40
Consider the following position function. find (a) the velocity and the speed of the object and (b) the acceleration of the object. bold r left parenthesis t right parenthesisr(t)equals=left angle 6 t superscript 4 baseline comma 2 t cubed right angle6t4,2t3 for tgreater than or equals≥0
Answers: 3

Physics, 22.06.2019 21:20
Astone is dropped from the upper observation deck of a tower, 250 m above the ground. (assume g = 9.8 m/s2.) (a) find the distance (in meters) of the stone above ground level at time t. h(t) = (b) how long does it take the stone to reach the ground? (round your answer to two decimal places.) s (c) with what velocity does it strike the ground? (round your answer to one decimal place.) m/s (d) if the stone is thrown downward with a speed of 2 m/s, how long does it take to reach the ground? (round your answer to two decimal places.) s
Answers: 3

Physics, 22.06.2019 23:40
Which type of energy is transferred by convection currents?
Answers: 2
You know the right answer?
When 25.9 j was added as heat to a particular ideal gas, the volume of the gas changed from 41.0 cm3...
Questions

Mathematics, 22.04.2020 11:58

Mathematics, 22.04.2020 11:58

Biology, 22.04.2020 11:58

Mathematics, 22.04.2020 11:58

Mathematics, 22.04.2020 11:58


History, 22.04.2020 11:58

Spanish, 22.04.2020 11:58






Mathematics, 22.04.2020 11:59

Mathematics, 22.04.2020 11:59



Mathematics, 22.04.2020 12:00

English, 22.04.2020 12:01

History, 22.04.2020 12:02

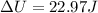
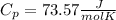
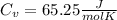

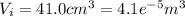

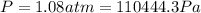
 .
.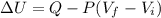 ⇒
⇒ 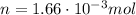
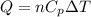 ⇒
⇒ 
 ⇒
⇒