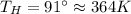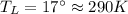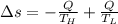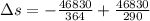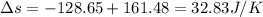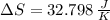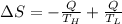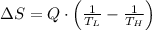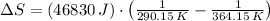Physics, 30.09.2019 23:30 noellelovebug1214
Suppose that there are two very large reservoirs of water, one at a temperature of 91.0 °c and one at a temperature of 17.0 °c. these reservoirs are brought into thermal contact long enough for 46830 j of heat to flow from the hot water to the cold water. assume that the reservoirs are large enough so that the temperatures do not change significantly. what is the total change in entropy resulting from this heat exchange between the hot water and the cold water?

Answers: 3


Another question on Physics

Physics, 22.06.2019 10:50
If jerome is swinging on a rope and transferring energy from gravitational potential energy to kinetic energy, is being done.
Answers: 3

Physics, 22.06.2019 15:30
What are the similarities & differences between a thermistor and a light dependent resistor in physics?
Answers: 2

Physics, 23.06.2019 01:30
Aball is thrown vertically upwards from the top of a tower with a speed of 100m/s.it strikes the pound near the base of the tower after 25sec . the height of the tower is
Answers: 3

Physics, 23.06.2019 02:20
What are known as the properties of substances that describe a substance et does not change that substances?
Answers: 1
You know the right answer?
Suppose that there are two very large reservoirs of water, one at a temperature of 91.0 °c and one a...
Questions

History, 10.11.2020 18:50

Social Studies, 10.11.2020 18:50

Mathematics, 10.11.2020 18:50

Mathematics, 10.11.2020 18:50

Mathematics, 10.11.2020 18:50


Mathematics, 10.11.2020 18:50


Chemistry, 10.11.2020 18:50

Mathematics, 10.11.2020 18:50

Mathematics, 10.11.2020 18:50



English, 10.11.2020 18:50


English, 10.11.2020 18:50