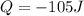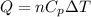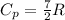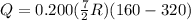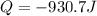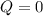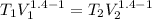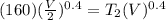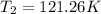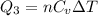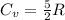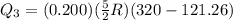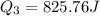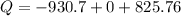Acylinder with a piston contains 0.200 mol of nitrogen at 1.50×105 pa and 320 k . the nitrogen may be treated as an ideal gas. the gas is first compressed isobarically to half its original volume. it then expands adiabatically back to its original volume, and finally it is heated isochorically to its original pressure. find the heat added to the gas during the final heating. find the internal-energy change of the gas during the final heating.

Answers: 3


Another question on Physics

Physics, 22.06.2019 00:30
Order the sequence of ideas that lead to marie curies discovery of radioactive elements number the events in chronological order starting with the oldest
Answers: 2


Physics, 22.06.2019 12:30
Aboy with a mass 25 kg climbs into a small tree. he sits on a branch that is 2.o m above to the ground.what is his gravitational potential energy above the ground?
Answers: 1

Physics, 22.06.2019 15:30
The voltage applied across a given parallel-plate capacitor is doubled. how is the energy stored in the capacitor affected?
Answers: 2
You know the right answer?
Acylinder with a piston contains 0.200 mol of nitrogen at 1.50×105 pa and 320 k . the nitrogen may b...
Questions





Biology, 25.06.2019 00:40

English, 25.06.2019 00:40

Biology, 25.06.2019 00:40










Mathematics, 25.06.2019 00:40