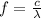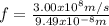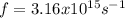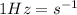Our understanding of the h atom will us learn about atoms with more electrons. the n =1 electron energy level of a h atom has an energy of 2.18 10–18 j. (a) what is the energy of the n = 5 level? (b) calculate the wavelength and frequency of a photon emitted when an electron jumps down from n = 5 to n = 1 in a h atom.

Answers: 1


Another question on Physics

Physics, 21.06.2019 20:20
Copper has free electrons per cubic meter. a 71.0-cm length of 12-gauge copper wire that is 2.05 mm in diameter carries 4.85 a of current. (a) how much time does it take for an electron to travel the length of the wire? (b) repeat part (a) for 6-gauge copper wire (diameter 4.12 mm) of the same length that carries the same current. (c) generally speaking, how does changing the diameter of a wire that carries a given amount of current affect the drift velocity of the electrons in the wire?
Answers: 2

Physics, 22.06.2019 05:00
Which of the following is the result of the nuclear weak force? the instability of large nuclei the repelling force between positively charged protons the structure of the atom certain types of nuclear decay
Answers: 2


You know the right answer?
Our understanding of the h atom will us learn about atoms with more electrons. the n =1 electron en...
Questions




Computers and Technology, 21.02.2020 21:25


Mathematics, 21.02.2020 21:25


Biology, 21.02.2020 21:25



Chemistry, 21.02.2020 21:25



History, 21.02.2020 21:25

Mathematics, 21.02.2020 21:25





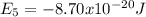
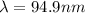 ,
, 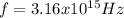
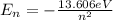 (1)
(1)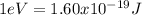
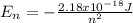
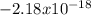 represents the energy of the ground state¹ and n is the principal quantum number.
represents the energy of the ground state¹ and n is the principal quantum number.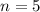 :
: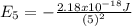
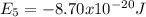
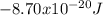 .
. 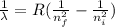 (2)
(2)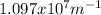
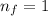 and
and 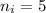 :
: 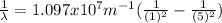
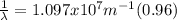
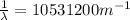
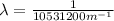
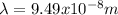
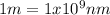
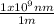
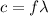 (3)
(3)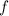 :
: