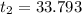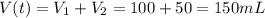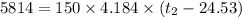Physics, 08.10.2019 04:00 Kingdcn6261
A100.0 ml sample of 1.020 m hcl is mixed with a 50.0 ml sample of 2.040 m naoh in a styrofoam cup. if both solutions were initially at 24.53°c, and the enthalpy of the neutralization reaction is −57 kj/mole of h2o formed, what is the final temperature of the mixture? assume that the solution has a density of 1.00 g/ml and a specific heat of 4.184 j/g°c, and that the styrofoam cup has an insignificant heat capacity.

Answers: 2


Another question on Physics

Physics, 21.06.2019 21:00
A150 w lamp emits light of wavelength 590 nm uniformly in all directions. what is the photon flux (photons per unit area per unit time) on a small screen at a distance 2.3 m from the lamp? assume the photons are uniformly distributed over the surface of a sphere of radius 2.3 m.
Answers: 2

Physics, 22.06.2019 05:30
Suppose you have three polarizing filters, with the second at an angle of 42∘ to the first and the third at an angle of 90∘ to the first. by what perfect will the original intensity of unpolarized light be reduced to after passing through all three filters?
Answers: 2

Physics, 22.06.2019 12:20
What is the coefficient of kinetic friction μk between the block and the tabletop?
Answers: 1

Physics, 22.06.2019 19:30
Listed below are the measured radiation absorption rates (in w/kg) corresponding to 11 cell phones. use the given data to construct a boxplot and identify the 5-number summary. 1.16 0.85 0.69 0.75 0.95 0.93 1.18 1.17 1.42 0.54 0.57 the 5-number summary is nothing, nothing, nothing, nothing, and nothing, all in w/kg. (use ascending order. type integers or decimals. do not round.)
Answers: 3
You know the right answer?
A100.0 ml sample of 1.020 m hcl is mixed with a 50.0 ml sample of 2.040 m naoh in a styrofoam cup. i...
Questions


Mathematics, 24.11.2019 05:31

History, 24.11.2019 05:31




Biology, 24.11.2019 05:31




Mathematics, 24.11.2019 05:31


Mathematics, 24.11.2019 05:31

Mathematics, 24.11.2019 05:31


Mathematics, 24.11.2019 05:31



Mathematics, 24.11.2019 05:31

Mathematics, 24.11.2019 05:31