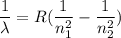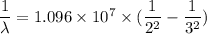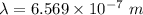The energy of the electron in a hydrogen atom can be calculated from the bohr formula: in this equation stands for the rydberg energy, and stands for the principal quantum number of the orbital that holds the electron. (you can find the value of the rydberg energy using the data button on the aleks toolbar.) calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with to an orbital with . round your answer to significant digits.

Answers: 3


Another question on Physics

Physics, 21.06.2019 20:20
To create electromagnetic induction, which of the following has to move? a. a wire b. a magnet c. a transformer d. either a or b
Answers: 2

Physics, 22.06.2019 04:20
The free body diagram shows a box being pulled to the left up a 25-degree incline. the magnitude of the normal force is
Answers: 1

Physics, 22.06.2019 21:00
Which of the following statements comparing electron microscopy and light microscopy is false? which of the following statements comparing electron microscopy and light microscopy is false? both the electron microscope and the light microscope use the same wavelengths for illumination. images produced by light microscopes can be in color, whereas electron microscope images are black and white unless they are artificially colored. the electron microscope has greater resolution than the light microscope. electron microscopes can allow examination of viruses and internal cell structures, whereas light microscopes are limited to objects that are 0.5 micrometers and larger. request answer
Answers: 2

Physics, 23.06.2019 01:00
2. circled insects have mutations, or changes to their dna. how many of the offspring insects in this generation have mutations?
Answers: 2
You know the right answer?
The energy of the electron in a hydrogen atom can be calculated from the bohr formula: in this equa...
Questions





Mathematics, 06.11.2020 01:00

English, 06.11.2020 01:00




Mathematics, 06.11.2020 01:00





Mathematics, 06.11.2020 01:00

Geography, 06.11.2020 01:00




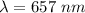
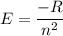 .............(1)
.............(1)