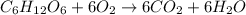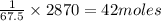Physics, 11.10.2019 03:30 student0724
Assume that the complete combustion of one mole of glucose, a monosaccharide, to carbon dioxide and water liberates 2870 kj of energy (δ°′=−2870 kj/mol ). if the energy generated by the combustion of glucose is entirely converted to the synthesis of a hypothetical compound x, calculate the number of moles of the compound that could theoretically be generated. use the value δ°′compound x=−67.5 kj/mol . round your answer to two significant figures.

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:30
Two technicians are discussing a resistance measurement between the can-h and can-l wires. technician a says this measurement should be done with the ignition switch in the "run" position. technician b states that a measurement of 0 ohms indicates an open in the network. which technician is correct?
Answers: 3

Physics, 22.06.2019 09:30
Gasoline comes from petroleum, which is made from ancient living things. petroleum, therefore, contains a type of energy. a. heat b. nuclear c. biological d. chemical potential
Answers: 2

Physics, 22.06.2019 10:00
If a stone with an original velocity of 0 is falling from a ledgeand takes 8 seconds to hoybthe ground whays the final velocity of the stone
Answers: 2

You know the right answer?
Assume that the complete combustion of one mole of glucose, a monosaccharide, to carbon dioxide and...
Questions

History, 22.06.2019 09:10

Mathematics, 22.06.2019 09:10

Mathematics, 22.06.2019 09:10



Mathematics, 22.06.2019 09:10



Mathematics, 22.06.2019 09:10


Mathematics, 22.06.2019 09:10





Mathematics, 22.06.2019 09:10

Chemistry, 22.06.2019 09:10



Mathematics, 22.06.2019 09:10