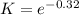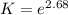The equation represents the decomposition of a generic diatomic element in its standard state. 12x2(g)⟶x(g) 12x2(g)⟶x(g) assume that the standard molar gibbs energy of formation of x(g) is 5.24 kj·mol−15.24 kj·mol−1 at 2000. k and −65.59 kj·mol−1−65.59 kj·mol−1 at 3000. k. determine the value of kk (the thermodynamic equilibrium constant) at each temperature. assuming that δh∘rxn is independent of temperature, determine the value of δh∘rxn from this data.

Answers: 3


Another question on Physics

Physics, 21.06.2019 23:30
To do work, this truck uses energy stored in chemical fuel and an electrical battery. how much total energy does this truck put out?
Answers: 1

Physics, 22.06.2019 09:30
Asap i'm in class rn a 1,000-kg car is traveling 20 m/s on a flat stretch of road. it gets to a hill and coasts uphill until it stops. how high up the hill does the car travel? givens: equation: 1/2mv2initial=mghfinal solve for h. plug & chug, label.
Answers: 2

Physics, 22.06.2019 12:30
Write a full page that sumerizes thermodynamics it’s from the website visionlearnig
Answers: 1

Physics, 22.06.2019 12:50
The heliocentric and the geocentric models of the solar system included these central principles
Answers: 1
You know the right answer?
The equation represents the decomposition of a generic diatomic element in its standard state. 12x2(...
Questions

Mathematics, 05.05.2020 22:35

Mathematics, 05.05.2020 22:35

Engineering, 05.05.2020 22:35

Biology, 05.05.2020 22:35

Mathematics, 05.05.2020 22:35



Chemistry, 05.05.2020 22:35


Social Studies, 05.05.2020 22:36

Health, 05.05.2020 22:36

Mathematics, 05.05.2020 22:36

History, 05.05.2020 22:36

English, 05.05.2020 22:36






Mathematics, 05.05.2020 22:36