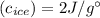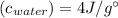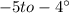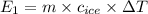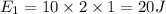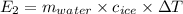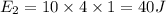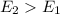Physics, 23.10.2019 19:00 lizzbugg9880
The specific heat of ice is about 2 j/g◦c while for liquid water the specific heat is about 4 j/g◦c. the energy required to raise the temperature of 10 g of ice from −5 to −4◦c is the energy required to raise the temperature of 10 g of liquid water from +4 to +5◦c. 1. greater than 2. less than 3. the same as

Answers: 1


Another question on Physics

Physics, 22.06.2019 03:50
A30 kg weight lies on top of a massless piston of area a = 0.01 m2 the exterior air is at a (constant) p =1 atm and t = 27 c. the interior gas is 0.4 moles of (ideal) n2 and it has initial temperature 27.00 degrees c. 1. what is the initial pressure in the interior? a. 29.4 kpa b. 130.7 kpa c. 101.3 kpa the next three questions concern what happens when an amount of heat q is slowly added to the interior, raising the piston by 1 mm and raising the interior temperature to 27.40 c
Answers: 3

Physics, 22.06.2019 12:00
Under the action of a constant force an object accelerates at 7.8 m/s2. what will the acceleration be if (a) the force is halved? (b) the object's mass is halved? (c) the force and the object's mass are both halved? (d) the force is halved and the object's mass is doubled?
Answers: 3

Physics, 22.06.2019 14:30
Which compound is held together by the electrostatic force between two ions? a. co2 b. cci4 c. h2s d. mgf2
Answers: 1

Physics, 22.06.2019 21:50
Determine the moment of the force f about an axis extending between a and c. express the result as a cartesian vector. a is at the origin, c is at (4,3,0) and f = (4i + 12j -3k) lb coming out of b (4,3,-2).
Answers: 2
You know the right answer?
The specific heat of ice is about 2 j/g◦c while for liquid water the specific heat is about 4 j/g◦c. t...
Questions




Computers and Technology, 24.06.2021 23:00

Mathematics, 24.06.2021 23:00

Mathematics, 24.06.2021 23:00

Mathematics, 24.06.2021 23:00






Physics, 24.06.2021 23:00


Mathematics, 24.06.2021 23:00

Health, 24.06.2021 23:00


Mathematics, 24.06.2021 23:00