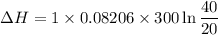One mole of a gas is placed in a closed system with a 20 l vessel initially at t = 300 k. the vessel is then isothermally expanded to 40 l. the gas follows the equation of state: p = rt/v + a/v2 where a = 240 l2 · atm/mol2 and r = 0.08206 l · atm/ mol · k. a. derive an expression relating (dh/dv)t to measurable properties. b. find dh for the gas in this process.

Answers: 2


Another question on Physics

Physics, 22.06.2019 06:20
Part 1: a magnetic levitation or maglev train rides rails without touching them. explain how this works using your data. include the appropriate magnet drawing in your answer. part 2: two objects are near a bar magnet. one is about 1 cm away, while the other is 6 cm away. compare and contrast the magnetic force that affects each object. use your data to answer the question
Answers: 1

Physics, 22.06.2019 09:10
The diagram shows a series of volcanic island and a hot spot determine the direction of movement of the tectonic plate that for the island
Answers: 2

Physics, 22.06.2019 13:50
9.98 kg of r-134a at 300 kpa fills a rigid container whose volume is 14 l. determine the temperature and total enthalpy in the container. the container is now heated until the pressure is 600 kpa. determine the temperature and total enthalpy when the heating is completed. use data from the steam tables.
Answers: 1

Physics, 22.06.2019 18:00
Gabby calls her cousin who lives in a different state and tells her to turn her radio to channel 98.7 so they can listen to their favorite song that is playing. her cousin turns her radio to 98.7, but does not hear the same song gabby hears. which most likely explains why?
Answers: 1
You know the right answer?
One mole of a gas is placed in a closed system with a 20 l vessel initially at t = 300 k. the vessel...
Questions


History, 24.06.2019 22:00




English, 24.06.2019 22:00

History, 24.06.2019 22:00

Biology, 24.06.2019 22:00

Computers and Technology, 24.06.2019 22:00





English, 24.06.2019 22:10

Mathematics, 24.06.2019 22:10




Computers and Technology, 24.06.2019 22:10

History, 24.06.2019 22:10

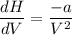
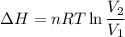 (ΔU=0)
(ΔU=0)