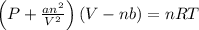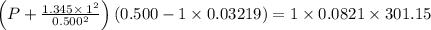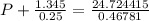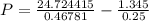Physics, 30.10.2019 00:31 miguel454545
If 1.00 mol of argon is placed in a 0.500-l container at 28.0 ∘c , what is the difference between the ideal pressure (as predicted by the ideal gas law) and the real pressure (as predicted by the van der waals equation)? for argon, a=1.345(l2⋅atm)/mol2 and b=0.03219l/mol.

Answers: 1


Another question on Physics


Physics, 21.06.2019 17:20
The specific heat of silver is 0.057 calories/gram°c. if 10.0 grams of silver were heated and the temperature of the sample changed by 20.0°c, how many calories of heat energy were absorbed by the sample?
Answers: 1

Physics, 22.06.2019 04:40
How is the gravitational force related to the distance between two objects?
Answers: 1

Physics, 22.06.2019 14:30
Lightning is an example of what phenomenon? a release of a large amount of energyan absorption of a large amount of energya natural electric circuita natural electric current
Answers: 1
You know the right answer?
If 1.00 mol of argon is placed in a 0.500-l container at 28.0 ∘c , what is the difference between th...
Questions


Mathematics, 25.02.2020 06:51







History, 25.02.2020 06:52


Mathematics, 25.02.2020 06:52

Biology, 25.02.2020 06:52


Mathematics, 25.02.2020 06:52

English, 25.02.2020 06:52


Social Studies, 25.02.2020 06:52


Mathematics, 25.02.2020 06:52

Mathematics, 25.02.2020 06:52