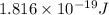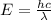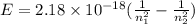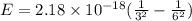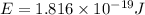An electron in a hydrogen atom undergoes a transition from the n = 3 level to the n = 6 level. to accomplish this, energy, in the form of light, must be absorbed by the hydrogen atom. calculate the energy of the light (in kj/photon) associated with this transition.

Answers: 1


Another question on Physics

Physics, 22.06.2019 05:00
Wavelength,frequency,and energy are related.what happens to a wave as it’s wavelength gets shorter?
Answers: 2

Physics, 22.06.2019 12:10
The average density of the planet uranus is 1.27 103 kg/m3. the ratio of the mass of neptune to that of uranus is 1.19. the ratio of the radius of neptune to that of uranus is 0.969. find the average density of neptune.
Answers: 1

Physics, 22.06.2019 18:10
A200-n force is applied to the foot-operated air pump. the return spring s exerts a 2.6-n·m moment on member oba for this position. determine the corresponding compression force c in the cylinder bd. if the diameter of the piston in the cylinder is 40 mm, estimate the air pressure generated for these conditions. state any assumptions. enter a positive number for the compression force c.
Answers: 2

Physics, 22.06.2019 20:00
What is the name of the perceived change in a sound wave’s frequency due to motion between the observer and the sound source?
Answers: 1
You know the right answer?
An electron in a hydrogen atom undergoes a transition from the n = 3 level to the n = 6 level. to ac...
Questions

Mathematics, 02.09.2020 06:01

Social Studies, 02.09.2020 06:01



Mathematics, 02.09.2020 06:01

Mathematics, 02.09.2020 06:01


Mathematics, 02.09.2020 06:01

Mathematics, 02.09.2020 06:01

Geography, 02.09.2020 06:01

Mathematics, 02.09.2020 06:01

Mathematics, 02.09.2020 06:01

Mathematics, 02.09.2020 06:01


Mathematics, 02.09.2020 06:01


Advanced Placement (AP), 02.09.2020 06:01

Computers and Technology, 02.09.2020 06:01

Health, 02.09.2020 06:01

English, 02.09.2020 06:01