
A25.0-g sample of copper at 363 k is placed in i 00.0 g of water at 293 k. the copper and water quickly come to the sa me temperature by the process of heat transfer from copper to water. calculate the final temperature of the water. the molar heat capacity of copper is 24.5 j · k-1 ·mol-1 and that of water is 75.2 1 · k -1 -mol-1•

Answers: 2


Another question on Physics

Physics, 22.06.2019 03:00
1. a net force of 100 newton’s is applied to a wagon for 5 seconds. this causes the wagon to undergo a change in momentum of
Answers: 2

Physics, 22.06.2019 07:20
If the ama of the inclined plane below is 2, calculate the ima and efficiency. ima = efficiency =
Answers: 1

Physics, 23.06.2019 05:30
Which form of pure carbon is so hard that it can be used in cutting tools?
Answers: 1

Physics, 23.06.2019 06:00
How can you improve the percent yield of copper in this lab? check all possible strategies that will you do so. measure reactants precisely. stir to the reaction take place. chill the reactants before combining them. wait patiently until the reaction completes. make sure to recover as much copper as possible.
Answers: 1
You know the right answer?
A25.0-g sample of copper at 363 k is placed in i 00.0 g of water at 293 k. the copper and water quic...
Questions



Computers and Technology, 24.06.2019 10:30













English, 24.06.2019 10:30

Mathematics, 24.06.2019 10:30


History, 24.06.2019 10:30

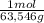 ×mass×ΔT
×mass×ΔT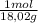 ×mass×ΔT
×mass×ΔT

