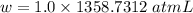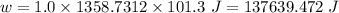Answers: 3


Another question on Physics

Physics, 22.06.2019 00:00
Jackson is designing a new heater, and he wants to experiment with different thermally conductive materials. which of these materials would be useful in conducting heat?
Answers: 1


Physics, 22.06.2019 14:30
Explain what it means to view something from a frame of reference. provide an example that illustrates your explanation. (4 points)
Answers: 1

Physics, 22.06.2019 14:50
Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = constant. the initial volume is 0.1 m3 and the work for the process is 121.14 kj. assuming ideal gas behavior, and neglecting kinetic and potential energy effects, determine heat transfer, in kj, and the entropy change, in kj/s. show the process on a t-s diagram.
Answers: 2
You know the right answer?
Calculate the amount of pv work done on the surroundings when 1.00 kg of h2o absorbs energy from sun...
Questions




Mathematics, 07.06.2021 23:10

Chemistry, 07.06.2021 23:10

World Languages, 07.06.2021 23:10



Chemistry, 07.06.2021 23:10

Mathematics, 07.06.2021 23:10


Mathematics, 07.06.2021 23:10

History, 07.06.2021 23:10

Mathematics, 07.06.2021 23:10


Mathematics, 07.06.2021 23:10

Mathematics, 07.06.2021 23:10


Mathematics, 07.06.2021 23:10

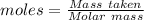
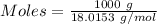
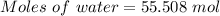
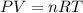
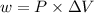
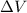 is the change in volume
is the change in volume