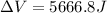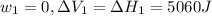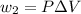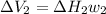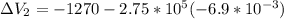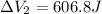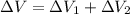Physics, 02.11.2019 07:31 chris018107
Find the total change in the internal energy of a gas that is subjected to the following two-step process. in the first step the gas is made to go through isochoric heating until 5460 j of heat is transferred into the gas and its pressure is 3.72 ✕ 105 pa. in the second step it is subjected to isobaric compression until its volume decreases by 7.50 ✕ 10−3 m3 and 1220 j of heat is transferred out of the gas. what is the total change in internal energy of this j?

Answers: 1


Another question on Physics

Physics, 21.06.2019 17:10
The dissociation energy of a molecule is the energy required tobreak apart the molecule into its separate atoms. the dissociationenergy for a particular molecule is 5.48 x 10-18 j.suppose that this energy is provided by a single photon. determinethe (a) wavelength and (b) frequency of the photon
Answers: 1

Physics, 22.06.2019 09:30
Need asap ‼️ 20 pts which gravitational force field diagram is drawn correctly? (answers in pictures below)
Answers: 1


Physics, 23.06.2019 08:10
Ais a multilevel way of grouping items based on common properties among them. a. memory b. conceptual hierarchy c. schema d. semantic network
Answers: 1
You know the right answer?
Find the total change in the internal energy of a gas that is subjected to the following two-step pr...
Questions


English, 10.06.2021 21:00






Mathematics, 10.06.2021 21:00

Mathematics, 10.06.2021 21:00

Mathematics, 10.06.2021 21:00

English, 10.06.2021 21:00





Social Studies, 10.06.2021 21:00




Mathematics, 10.06.2021 21:00