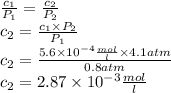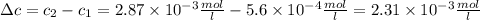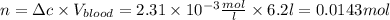The solubility of n2 in blood at 37°c and a partial pressure of 0.80 atm is 5.6 ✕ 10−4 mol·l−1. a deep-sea diver breathes compressed air with a partial pressure of n2 equal to 4.1 atm. assume that the total volume of blood in this diver's body is 6.2 l. calculate the amount of n2 gas released (in liters) when the diver returns to the surface of water, where the partial pressure of n2 is 0.80 atm. (2 sig fig)

Answers: 3


Another question on Physics

Physics, 21.06.2019 16:20
A1,200 kg car is accelerated at 3.7 m/s2. what force was needed to produce this acceleration? a. 4,440 n b. 324.3 n c. 4,388 n d. 304.3 n
Answers: 2

Physics, 22.06.2019 02:50
Ammonia enters the expansion valve of a refrigeration system at a pressure of 10 bar and a temperature of 18oc and exits at 6.0 bar. the refrigerant undergoes a throttling process. determine the temperature, in oc, and the quality of the refrigerant at the exit of the expansion valve.
Answers: 3

Physics, 22.06.2019 10:00
During which interval are the persons represented by the graph not moving? a) 0 to 0.5 hrs b) 1.5 to 2 hrs c) 2 to 2.5 hrs d) 4.5 to 5 hrs
Answers: 1

Physics, 22.06.2019 14:30
What conclusion can be made based on the temperature of soil when the light hits the soil at 0°, 45°, and 90° angles in section 2 of the experiment? did your results support your hypothesis? why or why not?
Answers: 1
You know the right answer?
The solubility of n2 in blood at 37°c and a partial pressure of 0.80 atm is 5.6 ✕ 10−4 mol·l−1. a de...
Questions

Chemistry, 26.08.2019 18:30






English, 26.08.2019 18:30








Mathematics, 26.08.2019 18:30


History, 26.08.2019 18:30