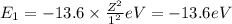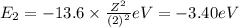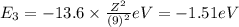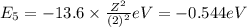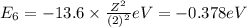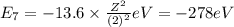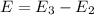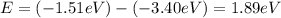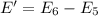Physics, 07.11.2019 03:31 darg3990rgp2t0r2
Of the following transitions in the bohr hydrogen atom, the transition results in the absorption of the highest-energy photon. n = 2 → n = 1 n = 2 → n = 3 n = 5 → n = 6 n = 7 → n = 5

Answers: 2


Another question on Physics


Physics, 21.06.2019 23:30
The pressure, volume, and temperature of a mole of an ideal gas are related by the equation pv = 8.31t, where p is measured in kilopascals, v in liters, and t in kelvins. use differentials to find the approximate change in the pressure if the volume increases from 14 l to 14.6 l and the temperature decreases from 375 k to 370 k. (round the answer to two decimal places.)
Answers: 3

Physics, 22.06.2019 10:00
During which interval are the persons represented by the graph not moving? a) 0 to 0.5 hrs b) 1.5 to 2 hrs c) 2 to 2.5 hrs d) 4.5 to 5 hrs
Answers: 1

Physics, 22.06.2019 12:30
If you place a magnet under a clear dish, and sprinkle iron fillings over it, above what part of the magnet will most of the fillings gather
Answers: 2
You know the right answer?
Of the following transitions in the bohr hydrogen atom, the transition results in the absorption of...
Questions



Mathematics, 20.02.2021 05:30

English, 20.02.2021 05:30





Arts, 20.02.2021 05:30

Mathematics, 20.02.2021 05:30




History, 20.02.2021 05:30


Geography, 20.02.2021 05:30



Advanced Placement (AP), 20.02.2021 05:30

English, 20.02.2021 05:30

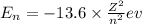
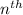 orbit will be,
orbit will be,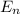 = energy of
= energy of