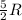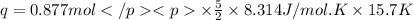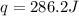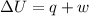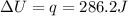A0.877 mol sample of n2(g) initially at 298 k and 1.00 atm is held at constant volume while enough heat is applied to raise the temperature of the gas by 15.7 k. assuming ideal gas behavior, calculate the amount of heat in joules required to affect this temperature change and the total change in internal energy, δ note that some books use δ as the symbol for internal energy instead of δ

Answers: 3


Another question on Physics

Physics, 22.06.2019 10:30
The uniform slender bar rests on a smooth horizon- tal surface when a force f is applied normal to the bar at point a. point a is observed to have an initial acceleration aa of 20 m/s2, and the bar has a corre- sponding angular acceleration of 18 rad /s2. deter- mine the distance b.
Answers: 1

Physics, 22.06.2019 13:10
Which of the following are used in an electrochemical cell? a. electrodes b. electrolyte c. terminals d. all of the above
Answers: 3

Physics, 22.06.2019 14:30
In order to do work, the force vector must be question 1 options: in a different direction than the acceleration vector. in a different direction than the displacement vector. in the same direction as the displacement vector and the motion. in the same direction as the acceleration vector.
Answers: 1

Physics, 22.06.2019 18:30
A1000-kg car is moving at 30 m/s around a horizontal unbanked curve whose diameter is 0.20 km. what is the magnitude of the friction force required to keep the car from sliding?
Answers: 3
You know the right answer?
A0.877 mol sample of n2(g) initially at 298 k and 1.00 atm is held at constant volume while enough h...
Questions





Mathematics, 19.04.2021 17:50


Mathematics, 19.04.2021 17:50

Mathematics, 19.04.2021 17:50




Social Studies, 19.04.2021 17:50

Mathematics, 19.04.2021 17:50

Mathematics, 19.04.2021 17:50


Mathematics, 19.04.2021 17:50

Mathematics, 19.04.2021 17:50

French, 19.04.2021 17:50

History, 19.04.2021 17:50

Mathematics, 19.04.2021 17:50

 is 286.2 J and 286.2 J respectively.
is 286.2 J and 286.2 J respectively.
 = Change in temperature = 15.7 K
= Change in temperature = 15.7 K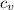 = heat capacity at constant volume of
= heat capacity at constant volume of 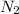 (diatomic molecule) =
(diatomic molecule) =