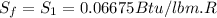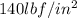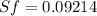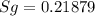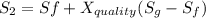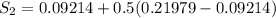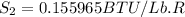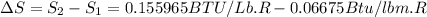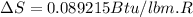Physics, 10.11.2019 06:31 GFJNIN9858
One lb of refrigerant 134a contained within a piston–cylinder assembly undergoes a process from a state where the temperature is 60f and the refrigerant is saturated liquid to a state where the pressure is 140 lbf/in 2 and quality is 50%. determine the change in specific entropy of the refrigerant, in btu/lbr. can this process be accomplished adiabatically?

Answers: 1


Another question on Physics

Physics, 22.06.2019 04:30
Asystem containing an ideal gas at a constant pressure of 1.22×10^5 pa gains 2140 j of heat. during the process, the internal energy of the system increases by 2320 j. what is the change in volume of the gas?
Answers: 3


Physics, 22.06.2019 15:10
What does si stand for,when referring to a system of measurement
Answers: 1

Physics, 22.06.2019 16:30
An astronaut in space cannot use a scale or balance to weigh objects because there is no gravity. but she does have devices to measure distance and time accurately. she knows her own mass is 77.4 kg , but she is unsure of the mass of a large gas canister in the airless rocket. when this canister is approaching her at 3.50 m/s , she pushes against it, which slows it down to 1.30 m/s (but does not reverse it) and gives her a speed of 2.60 m/s . what is the mass of the canister?
Answers: 1
You know the right answer?
One lb of refrigerant 134a contained within a piston–cylinder assembly undergoes a process from a st...
Questions


Chemistry, 25.01.2020 00:31



Computers and Technology, 25.01.2020 00:31




Computers and Technology, 25.01.2020 00:31









Business, 25.01.2020 00:31