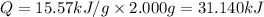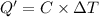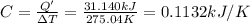Physics, 11.11.2019 20:31 clickbaitdxl
Constant-volume calorimeters are sometimes calibrated by running a combustion reaction of known δe and measuring the change in temperature. for example, the combustion energy of glucose is 15.57 kj/g. when a 2.000 g sample of glucose burns in a constant volume calorimeter, the calorimeter temperature increases from 21.45 to 23.34°c. find the total heat capacity of the calorimeter (in kj/k).

Answers: 1


Another question on Physics

Physics, 22.06.2019 11:50
Amoving electron has kinetic energy k1. after a net amount of work w has been done on it, the electron is moving one-quarter as fast in the opposite direction. (a) find w in terms of k1. (b) does your answer depend on the final direction of the electron's motion?
Answers: 2

Physics, 22.06.2019 14:10
Match these items. 1. coulombs __force 2. ohms __emf 3. centimeters __resistance 4. newtons __charge 5. volts __length
Answers: 1

Physics, 22.06.2019 14:30
Lightning is an example of what phenomenon? a release of a large amount of energyan absorption of a large amount of energya natural electric circuita natural electric current
Answers: 1

Physics, 22.06.2019 19:30
A47.2 g block of copper whose temperature is 480 k is placed in an insulating box with a 91.8 g block of lead whose temperature is 200 k. (a) what is the equilibrium temperature of the two-block system? (b) what is the change in the internal energy of the two-block system between the initial state and the equilibrium state? (c) what is the change in the entropy of the two-block system? the heat capacities of copper and lead are 386 j/kg·k and 128 j/kg·k, respectively.
Answers: 1
You know the right answer?
Constant-volume calorimeters are sometimes calibrated by running a combustion reaction of known δe a...
Questions

Social Studies, 14.05.2021 05:40


Mathematics, 14.05.2021 05:40

Biology, 14.05.2021 05:40


Geography, 14.05.2021 05:40


Arts, 14.05.2021 05:40





Social Studies, 14.05.2021 05:40

Health, 14.05.2021 05:40


Mathematics, 14.05.2021 05:40

Mathematics, 14.05.2021 05:40

Mathematics, 14.05.2021 05:40