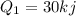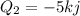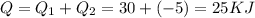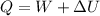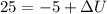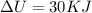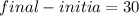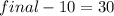Physics, 13.11.2019 18:31 leannamat2106
Water is being heated in a closed pan on top of a range while being stirred by a paddle wheel. during the process, 30 kj of heat is transferred to the water, and 5 kj of heat is lost to the surrounding air. the paddle-wheel work amounts to 5000 n m. determine the final energy of the system if its initial energy is 10 kj.

Answers: 1


Another question on Physics

Physics, 22.06.2019 02:00
Jasmine is diving off a 3-meter springboard. her height in meters above the water when she is x meters horizontally from the end of the board is approximated by the equation h=-x^2+3x+3. what is the maximum height jasmine will reach on her dive?
Answers: 3

Physics, 22.06.2019 05:00
Aperson walking 1 mile everyday for exercise, leaving her front porch at 9: 00 am and returning to her front porch at 9: 25 am. what is the total displacement of her daily walk? a) 1 mileb) 0c) 25 minutes d) none of the above
Answers: 1

Physics, 22.06.2019 14:30
A10nc charge sits at a point in space where the magnitude of the electric field is 1500 n/c. what will the magnitude of the field be if the 10 nc charge is replaced by a 20 nc charge? assume the system is big enough to consider the charges as small test charges.
Answers: 1

Physics, 22.06.2019 17:50
Which of the following best describes internal energy? a. the difference between the kinetic and potential energies of the particles in a system b. the sum of the kinetic and potential energies of the particles in a system c. the sum of the kinetic and thermal energies of the particles in a system d. the difference between the kinetic and thermal energies of the particles in a system
Answers: 2
You know the right answer?
Water is being heated in a closed pan on top of a range while being stirred by a paddle wheel. durin...
Questions

Biology, 23.01.2021 03:50


Mathematics, 23.01.2021 03:50



Mathematics, 23.01.2021 03:50

Biology, 23.01.2021 03:50

Mathematics, 23.01.2021 03:50


Mathematics, 23.01.2021 03:50




Mathematics, 23.01.2021 03:50

Chemistry, 23.01.2021 03:50

Mathematics, 23.01.2021 03:50


Mathematics, 23.01.2021 03:50

Mathematics, 23.01.2021 03:50