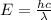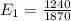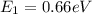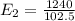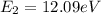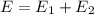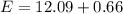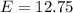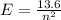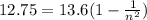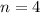Physics, 21.11.2019 21:31 queenbrebre2294
The electron in a hydrogen atom falls from an excited energy level to the ground state in two steps, causing the emission of photons with wavelengths of 1870 nm and 102.5 nm, respectively. what is the principal quantum number or shell of the initial excited energy level from which the electron falls?

Answers: 1


Another question on Physics

Physics, 21.06.2019 15:30
What defines the mass number of an isotope? a. the sum of the neutrons and protons b. the sum of the neutrons and electrons c. the number of neutrons d. the number of protons
Answers: 1

Physics, 22.06.2019 02:30
Agas contained within a piston-cylinder assembly undergoes three processes in series: process 12: compression with pv= constant from 1 bar and 1 liter to 4 bar. process 23: constant pressure expansion to 1 liter. process 31: constant volume calculate the pressure and volume at each state, and sketch the processes on a p-vdiagram labeled with pressure and volume values at each numbered stat
Answers: 2

Physics, 22.06.2019 08:50
The electronic structure or chlorine is 2.8.7 what is the electronic structure of fluorine?
Answers: 2

Physics, 22.06.2019 12:40
Find the equation for the plane through upper p 0 left parenthesis negative 4 comma negative 8 comma negative 5 right parenthesis perpendicular to the following line. xequalsnegative 4 minus t, yequalsnegative 8 plus 2 t, zequals3 t, minusinfinityless thantless thaninfinity
Answers: 2
You know the right answer?
The electron in a hydrogen atom falls from an excited energy level to the ground state in two steps,...
Questions





Social Studies, 29.08.2019 10:10


Mathematics, 29.08.2019 10:10

Mathematics, 29.08.2019 10:10

Mathematics, 29.08.2019 10:10

History, 29.08.2019 10:10

Mathematics, 29.08.2019 10:10

Mathematics, 29.08.2019 10:10

Arts, 29.08.2019 10:10


Mathematics, 29.08.2019 10:10


Social Studies, 29.08.2019 10:10

Biology, 29.08.2019 10:10


History, 29.08.2019 10:10