
In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid, or to measure the energy of a solution phase reaction. a student heats 69.12 grams of tungsten to 98.93 °c and then drops it into a cup containing 85.45 grams of water at 23.82 °c. she measures the final temperature to be 25.63 °c. the heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.56 j/°c. assuming that no heat is lost to the surroundings calculate the specific heat of tungsten.

Answers: 1


Another question on Physics

Physics, 21.06.2019 15:00
How much work would be needed to raise the payload from the surface of the moon (i.e., x = r) to an altitude of 5r miles above the surface of the moon (i.e., x = 6r)?
Answers: 2

Physics, 22.06.2019 04:20
Awave is produced in a rope. the wave has a speed of 33 m/s and a frequency of 22 hz.
Answers: 3

Physics, 22.06.2019 13:00
4. if you were an astronaut on the moon, what would you experience? what would you see from your perspective?
Answers: 1

Physics, 22.06.2019 18:00
Atank is filled with an ideal gas at 400 k and pressure of 1.00 atm . part a the tank is heated until the pressure of the gas in the tank doubles. what is the temperature of the gas?
Answers: 3
You know the right answer?
In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used t...
Questions

History, 12.10.2020 19:01







Mathematics, 12.10.2020 19:01

English, 12.10.2020 19:01


Biology, 12.10.2020 19:01


Social Studies, 12.10.2020 19:01




Mathematics, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01


Biology, 12.10.2020 19:01

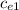 = 128.3 J / kg ° C
= 128.3 J / kg ° C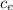 ΔT
ΔT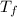 ) = (m₂
) = (m₂ 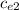 + C) (
+ C) (

