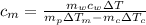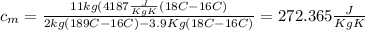Calculate the specific heat of a metal from the following data. a container made of the metal has a mass of 3.9 kg and contains 11 kg of water. a 2.0 kg piece of the metal initially at a temperature of 189°c is dropped into the water. the container and water initially have a temperature of 16.0°c, and the final temperature of the entire system is 18.0°c.

Answers: 3


Another question on Physics

Physics, 21.06.2019 19:30
The 20@kg wheel has a radius of gyration about its center g of kg = 300 mm. when it is subjected to a couple moment of m = 50 n # m, it rolls without slipping. determine the angular velocity of the wheel after its mass center g has traveled through a distance of sg = 20 m, starting from rest.
Answers: 3

Physics, 21.06.2019 22:00
Which type of microscope would allow the viewer to see ribosomes inside a cell?
Answers: 2

Physics, 22.06.2019 09:00
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 1

Physics, 22.06.2019 18:00
Sunidhi made a study chart about changes in states of matter. which headings best complete the chart?
Answers: 1
You know the right answer?
Calculate the specific heat of a metal from the following data. a container made of the metal has a...
Questions

Chemistry, 12.12.2019 17:31

Chemistry, 12.12.2019 17:31



English, 12.12.2019 17:31



History, 12.12.2019 17:31

Business, 12.12.2019 17:31

Biology, 12.12.2019 17:31

Mathematics, 12.12.2019 17:31



Advanced Placement (AP), 12.12.2019 17:31

Physics, 12.12.2019 17:31

History, 12.12.2019 17:31



Mathematics, 12.12.2019 17:31

Mathematics, 12.12.2019 17:31

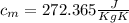
 = mass of the container = 3.9 kg
= mass of the container = 3.9 kg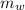 = mass of the water inside of the container= 11 kg
= mass of the water inside of the container= 11 kg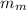 =mass of the metal= 2 kg
=mass of the metal= 2 kg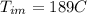 initital temperature of the metal
initital temperature of the metal initital temperature of the water
initital temperature of the water initital temperature of the container
initital temperature of the container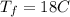 final equilibrium temperature
final equilibrium temperature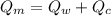
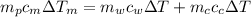
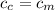
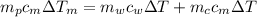
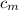 from the last expression we got:
from the last expression we got: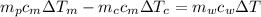
![c_m [m_p \Delta T_m -m_c \Delta T_c] =m_w c_w \Delta T](/tpl/images/0387/5562/73942.png)