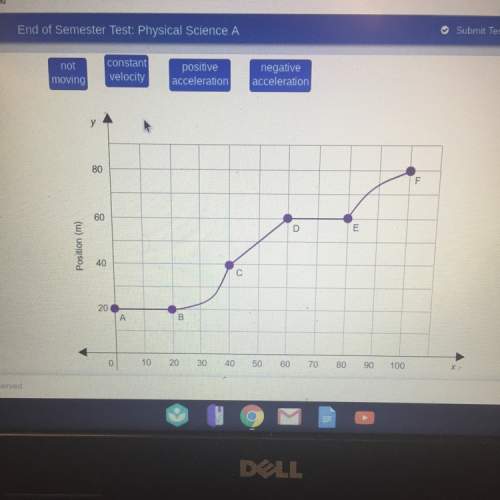
When the hydrogen atom makes the transition from the n=2 to the n=1 energy level, it emits a photon. this photon can be absorbed by to a singly ionized helium atom (he+) in its second energy level (ninitial=2), causing it to move to a higher energy level. to what final energy level number nfinal can the photon promote the helium ion?
note that he+ has two protons in its nucleus and a single electron.

Answers: 1


Another question on Physics

Physics, 22.06.2019 02:30
Gunpowder residue is most likely to show up where on a shooters hands
Answers: 1

Physics, 22.06.2019 12:00
An architect plans to use solar energy to heat the next house he designs. what principle of absorption and infrared energy can be applied to the design of the new house? how could she apply to those principals?
Answers: 2

Physics, 22.06.2019 13:00
The law of the conservation of momentum says that the total momentum of any closed and isolated system cannot
Answers: 1

Physics, 22.06.2019 21:00
Ais a machine that makes work easier when a single force is applied. a simple machine b compound machine c endangered machine d complex machine
Answers: 2
You know the right answer?
When the hydrogen atom makes the transition from the n=2 to the n=1 energy level, it emits a photon....
Questions






Mathematics, 30.07.2019 03:10


Mathematics, 30.07.2019 03:10



Mathematics, 30.07.2019 03:10

Mathematics, 30.07.2019 03:10

Mathematics, 30.07.2019 03:10

English, 30.07.2019 03:10

Mathematics, 30.07.2019 03:10





Computers and Technology, 30.07.2019 03:10

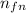 = 4
= 4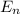 = - 13.606 / n² [eV]
= - 13.606 / n² [eV]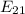 = - 13.606 [¼ -1/1]
= - 13.606 [¼ -1/1]