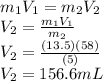Physics, 25.11.2019 21:31 KrishnaBalaram1235
Achemist must dilute 58.0 ml of 13.5 m aqueous silver nitrate (agno3)solution until the concentration falls to 5.00 m . he'll do this by adding distilled water to the solution until it reaches a certain final volume. calculate this final volume, in liters. round your answer to 3significant digits.

Answers: 1


Another question on Physics

Physics, 22.06.2019 06:00
Which of the following changes will result in a stronger electromagnet? a. using fewer coils of wire b. using a higher voltage c. using a shorter nail d. using a longer wire
Answers: 1

Physics, 22.06.2019 08:00
5g of ammonium nitrate was dissolved in 60g of water in an insulated container. the temperature at the start of the reaction was 23.0°c and at the end it was 19.0°c. calculate the energy absorbed by the reaction.
Answers: 3

Physics, 22.06.2019 17:30
Four objects each with charge +2.0×10−7c are located at the corners of a square whose sides are 2.0 m long. part a what quantities can be determined using this information? check all that apply. the electric force on a charged object placed at the center of the square. the mass of each object. the total electric potential energy of the system consisting of the four charged objects. part b find the electric force on a charged object placed at the center of the square.
Answers: 1

Physics, 22.06.2019 20:20
An electron is trapped at a defect in a crystal. the defect may be modeled as a one-dimensional, rigid-walled box of width 1.00 nm. (a) sketch the wavefunctions and probability densities for the n 1 and n 2 states. (b) for the n 1 state, nd the probability of nding the electron between x1 0.15 nm and x2 0.35 nm, where x 0 is the left side of the box. (c) repeat (b) for the n 2 state. (d) calculate the energies in electron volts of the n 1 and n 2 states
Answers: 1
You know the right answer?
Achemist must dilute 58.0 ml of 13.5 m aqueous silver nitrate (agno3)solution until the concentratio...
Questions







Physics, 04.08.2019 08:00






Biology, 04.08.2019 08:00

English, 04.08.2019 08:00

Computers and Technology, 04.08.2019 08:00

Mathematics, 04.08.2019 08:00




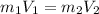
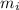 = Mass
= Mass 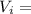 Volume
Volume 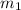 = 13.5M
= 13.5M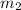 = 5M
= 5M 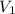 = 58.0mL
= 58.0mL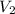 we have,
we have,