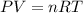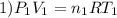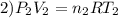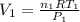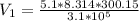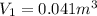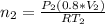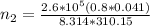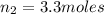Physics, 28.11.2019 01:31 nonispn606
Imagine a car tire that contains 5.1 moles of air when at a gauge pressure of 2.1×10^5n/m2 (the pressure above atmospheric pressure) and a temperature of 27 degrees c. the temperature increases to 37 degrees c, the volume decreases to 0.8 times the original volume, and the gauge pressure decreases to 1.6×10^5n/m2.
how many moles of air are left in the tire?

Answers: 2


Another question on Physics

Physics, 22.06.2019 15:00
What pressure difference is required between the ends of a 2.0-m-long, 1.0-mm-diameter horizontal tube for 40 c water to flow through it at an average speed of 4.0 m/s?
Answers: 1

Physics, 22.06.2019 17:40
Which component of the earth’s atmosphere is decreased due to photosynthesis?
Answers: 1

Physics, 22.06.2019 18:30
Asmall 12.00g plastic ball is suspended by a string in a uniform, horizontal electric field with a magnitude of 10^3 n/c. if the ball is in equilibrium when the string makes a 30 ° angle with the vertical, what is the net charge on the ball?
Answers: 1

Physics, 23.06.2019 03:30
As you lift an 88 n box straight upward you produce a power of 72 w. what is the speed of the box?
Answers: 2
You know the right answer?
Imagine a car tire that contains 5.1 moles of air when at a gauge pressure of 2.1×10^5n/m2 (the pres...
Questions



Mathematics, 27.04.2020 02:12

English, 27.04.2020 02:12





Mathematics, 27.04.2020 02:12





Mathematics, 27.04.2020 02:12


Mathematics, 27.04.2020 02:12

Mathematics, 27.04.2020 02:12

English, 27.04.2020 02:12

Medicine, 27.04.2020 02:12