
Physics, 02.12.2019 20:31 lavorisjonesjr1
Consider that 168.0 j of work is done on a system and 305.6 j of heat is extracted from the system. in the sense of the first law of thermodynamics, what is the value (including algebraic sign) of w, the work done by the system?

Answers: 1


Another question on Physics

Physics, 21.06.2019 17:40
Sarah and michelle work at a tech company that designs exercise apps. sarah tells michelle she has heard that james, their supervisor, interviewed with a competitor and may be leaving the company, which would open up a management position. what kind of power is sarah exerting?
Answers: 1


Physics, 22.06.2019 13:30
6–48 bananas are to be cooled from 24 to 138c at a rate of 215 kg/h by a refrigeration system. the power input to the refrigerator is 1.4 kw. determine the rate of cooling, in kj/ min, and the cop of the refrigerator. the specific heat of banana above freezing is 3.35 kj/kg·8c.
Answers: 3

Physics, 22.06.2019 19:00
The law of reflection states that the angle of reflection is equal to the angle of
Answers: 1
You know the right answer?
Consider that 168.0 j of work is done on a system and 305.6 j of heat is extracted from the system....
Questions



Computers and Technology, 28.06.2019 06:50

Mathematics, 28.06.2019 06:50


Biology, 28.06.2019 06:50

Physics, 28.06.2019 06:50

Mathematics, 28.06.2019 06:50

Mathematics, 28.06.2019 06:50


Mathematics, 28.06.2019 06:50

Geography, 28.06.2019 06:50






Social Studies, 28.06.2019 06:50


English, 28.06.2019 06:50

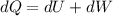
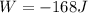 Work is done ON the system
Work is done ON the system 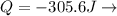 Heat is extracted FROM the system
Heat is extracted FROM the system

