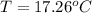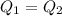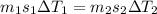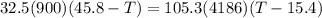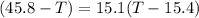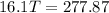Physics, 03.12.2019 01:31 Gghbhgy4809
A32.5 g cube of aluminum initially at 45.8 °c is submerged into 105.3 g of water at 15.4 °c. what is the final temperature of both substances at thermal equilibrium? (assume that the aluminum and the water are thermally isolated from everything else.)

Answers: 2


Another question on Physics

Physics, 22.06.2019 02:30
If a refrigerator is a heat pump that follows the first law of thermodynamics, how much heat was removed from food inside of the refrigerator if it released 380j of energy to the room?
Answers: 1

Physics, 22.06.2019 03:00
An electric current is flowing through the cord below.what will happen to this current if a magnet is brought near the cord? a. the electric current will stop flowing. b. it will exert a force on the voltage. c. the resistance of the wire will decrease. d. it will exert a force on the electric current.
Answers: 2

Physics, 22.06.2019 10:00
What is the temperature in degrees celsius of a substance with a tempature of 49k
Answers: 2

Physics, 22.06.2019 15:30
Ametal ring 4.20 cm in diameter is placed between the north and south poles of large magnets with the plane of its area perpendicular to the magnetic field. these magnets produce an initial uniform field of 1.12 t between them but are gradually pulled apart, causing this field to remain uniform but decrease steadily at 0.240 t/s . (a) what is the magnitude of the electric field induced in the ring? (b) in which direction (clockwise or counterclockwise) does the current flow as viewed by someone on the south pole of the magnet?
Answers: 2
You know the right answer?
A32.5 g cube of aluminum initially at 45.8 °c is submerged into 105.3 g of water at 15.4 °c. what is...
Questions


Biology, 14.02.2020 03:47






Mathematics, 14.02.2020 03:47

Mathematics, 14.02.2020 03:47




Computers and Technology, 14.02.2020 03:47







History, 14.02.2020 03:47