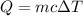Physics, 03.12.2019 18:31 gwoodbyrne
7. a block of copper of unknown mass has an initial temperature of 65.4oc. the copper is immersed in a beaker containing 95.7g of water at 22.7oc. when the two substances reach thermal equilibrium, the final temperature is 24.2oc. what is the mass of the copper block?

Answers: 2


Another question on Physics

Physics, 21.06.2019 20:20
An asteroid is discovered in a nearly circular orbit around the sun, with an orbital radius that is 2.47 times earth's. what is the asteroid's orbital period, its "year," in terms of earth years?
Answers: 1


Physics, 22.06.2019 14:00
Agraduated cylinder contains 63.0 ml of water. a piece of gold, which has a density of 19.3 g/ cm3, is added to the water and the volume goes up to 64.5 ml. calculate the mass in grams of the gold that was added to the water. explain how you got your answer.
Answers: 3

Physics, 22.06.2019 15:00
Holes drilled several kilometers into earth’s crust provide direct evidence about earth’s interior in the form of
Answers: 1
You know the right answer?
7. a block of copper of unknown mass has an initial temperature of 65.4oc. the copper is immersed in...
Questions

Mathematics, 03.07.2019 19:00


Advanced Placement (AP), 03.07.2019 19:00

Mathematics, 03.07.2019 19:00

Mathematics, 03.07.2019 19:00

History, 03.07.2019 19:00




English, 03.07.2019 19:00

Mathematics, 03.07.2019 19:00

Social Studies, 03.07.2019 19:00


Mathematics, 03.07.2019 19:00




Mathematics, 03.07.2019 19:00

Mathematics, 03.07.2019 19:00

 = Change in temperature
= Change in temperature