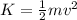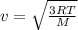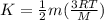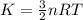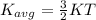Physics, 03.12.2019 19:31 guzmangisselle
Gas a has molecules with small mass. gas b has molecules with larger mass. they are at the same temperature.
how do the gases compare with respect to the average translational kinetic energy?
a)a has a larger average kinetic energy. b)b has a larger average kinetic energy. c)the gases have the same average kinetic energy.

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:30
Ais not a compound machine. a. bolt b. shovel c. handcart (dolly) d. see-saw (teeter totter)
Answers: 2

Physics, 22.06.2019 02:30
Explain the difference between each pair of concepts. a. frequency and relative frequency b. percentage and relative frequency a. select the correct choice below. a. frequency is the total number of observations in a data set. relative frequency is the number of times a particular distinct value occurs. b. frequency is the number of times a particular distinct value occurs. relative frequency is the ratio of the frequency of a value to the total number of observations. c. frequency is the total number of observations in a data set. relative frequency is the ratio of the number of times a particular distinct value occurs to the frequency. d. frequency is the number of times a particular distinct value occurs. relative frequency is the ratio of the frequency of two different values.
Answers: 3


Physics, 22.06.2019 08:30
Individuals who live below the poverty line get seriously ill more often than those who do not what could be the hidden variable in this situation?
Answers: 3
You know the right answer?
Gas a has molecules with small mass. gas b has molecules with larger mass. they are at the same temp...
Questions


Mathematics, 06.05.2020 00:23



Mathematics, 06.05.2020 00:23




Mathematics, 06.05.2020 00:23




Mathematics, 06.05.2020 00:23



Mathematics, 06.05.2020 00:23

Mathematics, 06.05.2020 00:23


Advanced Placement (AP), 06.05.2020 00:23

Mathematics, 06.05.2020 00:23