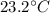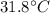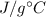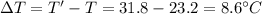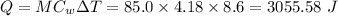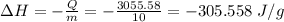In a coffee-cup calorimeter experiment, 10.00 g of a soluble ionic compound was added to the calorimeter contained 75.0 g h2o initially at 23.2°c. the final temperature of the solution was 31.8°c. what was the change in enthalpy for the dissolution of this compound?

Answers: 1


Another question on Physics

Physics, 21.06.2019 13:30
Me ! a 2 µc charge q1 and a 2 µc charge q2 are 0.3 m from the x-axis. a 4 µc charge q3 is 0.4 m from the y-axis. the distances d13 and d23 are 0.5 m. find the magnitude and direction of the resulting vector r. round your answer to the nearest tenth.
Answers: 2

Physics, 21.06.2019 13:50
Aboat that travels with constant speed of 6.10 m/s in still water is to go directly across a river. the current in the river flows at 1.95 m/s. (a) at what angle must the boat be steered?
Answers: 1

Physics, 21.06.2019 16:40
An airplane flying parallel to the ground undergoes two consecutive displacements. the first is 76 km at 39.8◦ west of north, and the second is 156 km at 59.9◦ east of north. what is the magnitude of the plane’s total displacement? answer in units of km. 020 (part 2 of 2) 10.0
Answers: 1

Physics, 22.06.2019 03:00
If a spring has a k value of 100 newtons per meter and it is stretched 0.50 meters, what is the restoring force of the spring?
Answers: 2
You know the right answer?
In a coffee-cup calorimeter experiment, 10.00 g of a soluble ionic compound was added to the calorim...
Questions


















Computers and Technology, 24.12.2019 04:31