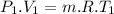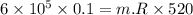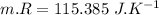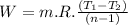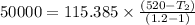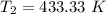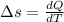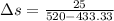Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = constant. the initial volume is 0.1 m3 and the work for the process is 50 kj. assuming ideal gas behavior, and neglecting kinetic and potential energy effects, determine heat transfer, in kj, and the entropy change, in kj/k.

Answers: 2


Another question on Physics

Physics, 21.06.2019 16:20
Two identical small charged spheres are a certain distance apart, and each one initially experiences an electrostatic force of magnitude f due to the other. with time, charge gradually leaks off of both spheres. when each of the spheres has lost half its initial charge, the magnitude of the electrostatic force will be
Answers: 1

Physics, 22.06.2019 00:30
Orange juice has a lower or higher viscosity than chocolate syrup
Answers: 2

Physics, 22.06.2019 04:00
Acompound machine is also called a machine. a. force b. simple c. complex d. directional
Answers: 1

You know the right answer?
Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = cons...
Questions

Mathematics, 23.04.2021 05:30




Social Studies, 23.04.2021 05:30


Mathematics, 23.04.2021 05:30

Mathematics, 23.04.2021 05:30

English, 23.04.2021 05:30


Mathematics, 23.04.2021 05:30



Mathematics, 23.04.2021 05:30



Mathematics, 23.04.2021 05:30


History, 23.04.2021 05:30

English, 23.04.2021 05:30

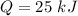
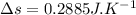
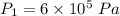 initial temperature,
initial temperature, 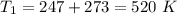 polytropic index,
polytropic index, 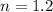 initial volume,
initial volume, 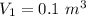 work done in the process,
work done in the process, 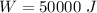
![Q=W[\frac{\gamma -n}{\gamma-1} ]](/tpl/images/0402/4036/a3071.png)
![Q=50\times[\frac{1.4-1.2}{1.4-1} ]](/tpl/images/0402/4036/3ed0d.png)