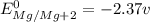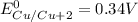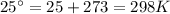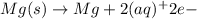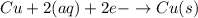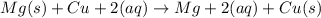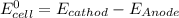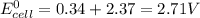Physics, 06.12.2019 19:31 xxleeciexx
Calculate the standard emf of a cell that uses the mg/mg2+ and cu/cu2+ half-cell reaction at 25°c. e o = v write the equation for the cell reaction that occurs under standard-state conditions. be sure to include the physical state of each species in the reaction.

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:30
A175 g lump of molten lead at its melting point (327 c) is placed into 55.0 g of water at 20.0 c. the specific heat of lead is 130.j/kg c and the hf of lead is 20,400 j/kg. when the lead and the water have reached equilibrium, what is the temperature of the mixture?
Answers: 3



Physics, 22.06.2019 06:50
What is the stall speed at sea level of an airplane that weights 10000 lbs., has a wing area of 300ft^2, and a maximum lift coefficient of 1.4? what is the stall speed if flaps that double cl,max are applied?
Answers: 1
You know the right answer?
Calculate the standard emf of a cell that uses the mg/mg2+ and cu/cu2+ half-cell reaction at 25°c. e...
Questions

Mathematics, 01.12.2020 22:50






Social Studies, 01.12.2020 22:50

History, 01.12.2020 22:50


Mathematics, 01.12.2020 22:50

Mathematics, 01.12.2020 22:50


Mathematics, 01.12.2020 22:50


Chemistry, 01.12.2020 22:50


Chemistry, 01.12.2020 22:50

Mathematics, 01.12.2020 22:50


Mathematics, 01.12.2020 22:50