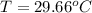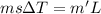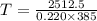Physics, 07.12.2019 00:31 herchellann302
You place an ice cube of mass 7.50×10−3kg and temperature 0.00∘c on top of a copper cube of mass 0.220 kg. all of the ice melts, and the final equilibrium temperature of the two substances is 0.00∘c. what was the initial temperature of the copper cube? assume no heat is exchanged with the surroundings.

Answers: 1


Another question on Physics

Physics, 22.06.2019 17:30
Does heating a metal wire increase or decrease its electrical resistance? why?
Answers: 1

Physics, 22.06.2019 18:00
By what primary heat transfer mechanism does the sun warm the earth?
Answers: 1


Physics, 23.06.2019 02:30
If jason ii needs 15 kw of power at 300 volts to operate, what amperage of current must flow through the cable?
Answers: 1
You know the right answer?
You place an ice cube of mass 7.50×10−3kg and temperature 0.00∘c on top of a copper cube of mass 0.2...
Questions


Mathematics, 16.02.2021 21:20

Computers and Technology, 16.02.2021 21:20

Mathematics, 16.02.2021 21:20

Mathematics, 16.02.2021 21:20






Mathematics, 16.02.2021 21:20


Mathematics, 16.02.2021 21:20

Mathematics, 16.02.2021 21:20



History, 16.02.2021 21:20

Chemistry, 16.02.2021 21:20

French, 16.02.2021 21:20