
Physics, 07.12.2019 02:31 jesusdelao
Consider the reaction: n2(g) + 3 f2(g) → 2 nf3(g) δh° = -249 kj and δs° = -278 j/k at 25°c calculate δg° and state whether the equilibrium composition should favor reactants or products at standard conditions.

Answers: 2


Another question on Physics

Physics, 21.06.2019 23:30
An object starts from rest at point f and speeds up continuously as it moves around an oval. a. choose a point about 1/8 th of the way around the oval from point f, and label it point g. draw a vector to represent the velocity of the object at point g. b. determine the change in velocity vector between points f and g.
Answers: 1

Physics, 22.06.2019 04:20
Calculate the capacitance of a system that stores 2.0 x 10^-10c of charge at 100.0 v. use c=q/v. a. 2.0 x 10^-12 f b. 2.0 x 10^-8 f c. 5.0 x 10^11 f d. 5.0 x 10^7 f
Answers: 1

Physics, 22.06.2019 07:30
Quantum mechanics applies to subatomic, atomic, nanometer-size, and micrometer-size systems. nanometer, micrometer, and kilometer-size systems. atomic, nanometer-size, and micrometer-size systems. subatomic, atomic, and nanometer-size systems.
Answers: 2

Physics, 22.06.2019 11:00
What is the rate of 12 liters of water moving through a water hose in 4.0 minutes?
Answers: 1
You know the right answer?
Consider the reaction: n2(g) + 3 f2(g) → 2 nf3(g) δh° = -249 kj and δs° = -278 j/k at 25°c calculat...
Questions




Mathematics, 22.04.2020 03:21


History, 22.04.2020 03:21

History, 22.04.2020 03:21






Mathematics, 22.04.2020 03:21

History, 22.04.2020 03:21



English, 22.04.2020 03:22

History, 22.04.2020 03:22


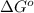 is -166156 J and equilibrium composition should favor products at standard conditions.
is -166156 J and equilibrium composition should favor products at standard conditions.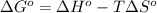
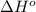 = standard enthalpy = -249 kJ = -249000 J
= standard enthalpy = -249 kJ = -249000 J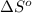 = standard entropy = -278 J/K
= standard entropy = -278 J/K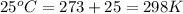

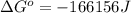
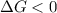 and reaction will be favored in the forward direction that means favored in products.A reaction to be non-spontaneous when
and reaction will be favored in the forward direction that means favored in products.A reaction to be non-spontaneous when 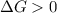 and reaction will be favored in the backward direction that means favored in reactants.
and reaction will be favored in the backward direction that means favored in reactants.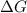 is less than zero that means the reaction is spontaneous and reaction will be favored in the forward direction that means favored in products.
is less than zero that means the reaction is spontaneous and reaction will be favored in the forward direction that means favored in products.

