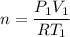Physics, 07.12.2019 03:31 ggpro4life3000
An ideal monatomic gas is contained in a vessel of constant volume 0.230 m3. the initial temperature and pressure of the gas are 300 k and 5.00 atm, respectively. the goal of this problem is to find the temperature and pressure of the gas after 23.0 kj of thermal energy is supplied to the gas.
(a) use the ideal gas law and initial conditions to calculate the number of moles of gas in the vessel.
mol
(b) find the specific heat of the gas.
j/k
(c) what is the work done by the gas during this process?
kj
(d) use the first law of thermodynamics to find the change in internal energy of the gas.
kj
(e) find the change in temperature of the gas.
k
(f) calculate the final temperature of the gas.
k
(g) use the ideal gas expression to find the final pressure of the gas.
atm

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:00
What is work? how much work is done to move an object 0 meters?
Answers: 1

Physics, 22.06.2019 06:30
2kg of refrigerant 134a undergoes a polytropic process in a piston-cylinder assembly from an initial state of saturated vapor at 2 bar to a final state of 12 bar, 80 degree c. a)determine the work for the process in kj. b)sketch the process on a p-v diagram.
Answers: 2

Physics, 22.06.2019 07:10
1. how much energy is needed to raise the temperature of 40.0 g of argon from 25c to 40c? the specific heat capacity of argon is 0.520 j/(g·k) 2a. 23.0 ml of 0.100 m hcl (standard) are added from a buret to neutralize 50.0 ml of an unknown basic solution. 2b. if the oh- produced in the previous reaction came from ca(oh)2, then what is the molarity of the ca(oh)2? 3.calculate the new freezing-point of a solution when 60.5 grams of cacl2 solute is dissolved in 0.612 kg of water. 4. what is the maximum number of moles of alcl3 that can be produced from 5.0 mol al and 6.0 mol cl2? 5. a sample of oxygen gas has a volume of 150 ml when its pressure is 0.923 atm. if the pressure is increased to 0.987 atm and the temperature remains constant, what will the new volume be? 6. nitrogen gas in a closed container at a temperature of 100.0 oc and 3.0 atm is heated to 300 oc. what is the pressure of the gas at the higher temperature?
Answers: 3

Physics, 22.06.2019 14:30
Which pair of quantities includes one quantity that increases as the other decreases during simple harmonic motion?
Answers: 1
You know the right answer?
An ideal monatomic gas is contained in a vessel of constant volume 0.230 m3. the initial temperature...
Questions

Mathematics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


Physics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

History, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


Physics, 20.09.2020 01:01

Health, 20.09.2020 01:01

Geography, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01


Physics, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01


Physics, 20.09.2020 01:01